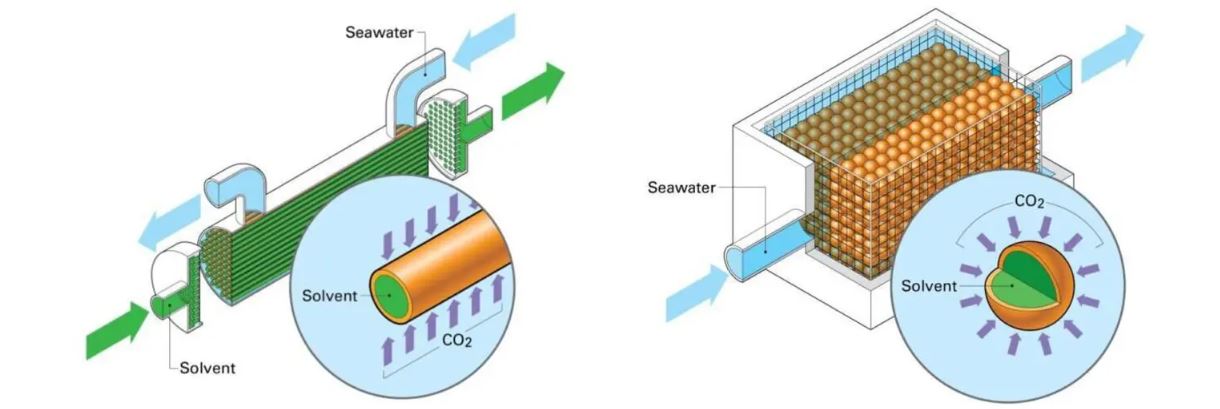
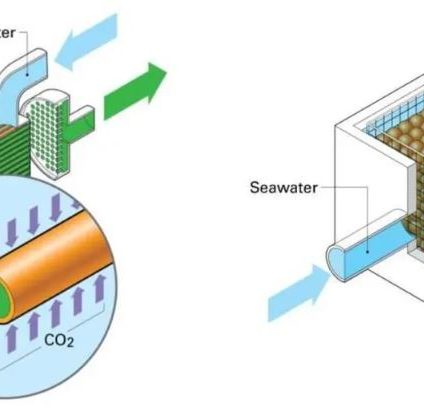
A team of researchers at the University of Pittsburgh has developed two special membrane contactors that use a sodium solution solvent to sequester carbon dioxide from the ocean (and increase its ability to absorb CO2 from the atmosphere)
Ocean absorbs 25% of man-made CO2
The oceans play a fundamental role in mitigating the effects of global warming on the Planet. They absorb up to 90% of the excess heat in the atmosphere, about 220-240 zettajoule (ZJ) each year. A huge amount of energy: with 2.2 ZJ you could heat the entire atmosphere of the Earth by 1 C. And they also absorb CO2: about 25% of the carbon dioxide that humanity generates every year. Limiting the increase in its concentration in the atmosphere and therefore the greenhouse effect. Because of this second quality, the seas are at the center of many studies to use them as a solution to climate change. An innovative method comes from the University of Pittsburgh and focuses on the direct ocean carbon capture.
How does the direct ocean carbon capture
The oceans are at the center of several attempts at geoengineering applications. One of the most common is to make the pH of the water more basic to increase its ability to absorb CO2 through enhanced weathering, which consists in the “enhanced disintegration” of silicate rocks, that is, in the acceleration of the natural process of disintegration of rocks due to atmospheric agents. Silicate-rich powder helps lower the pH.
The method developed in Pittsburgh reverses the argument: it consists in subtracting carbon dioxide from the ocean (and then storing it elsewhere), with the result of increasing its absorption capacity. How is the direct capture of CO2 from the ocean?
read also Carbon capture, the world’s fastest system has 99% efficiency
Researchers at the US University have tested two different types of membrane contactors, tools that are already commonly used today, for example, by the food industry to control the dosage of CO2 in beverages. Both use a solvent based on a sodium solution that reacts with seawater by separating CO2.
The first membrane contactor (a “encapsulated solvent“) consists of a cluster of tiny balls that host the solvent, the second (a “hollow fiber“) is formed by small full filaments of the solvent. In both cases, the specific shape serves to increase the contact surface between marine and solvent water and thus optimize the process of direct ocean carbon capture.