New research tries to exploit the energy released by the process of chemical adsorption of CO2 through "ionic" processes based on nature
A new nanogenerator to produce electricity from atmospheric CO2
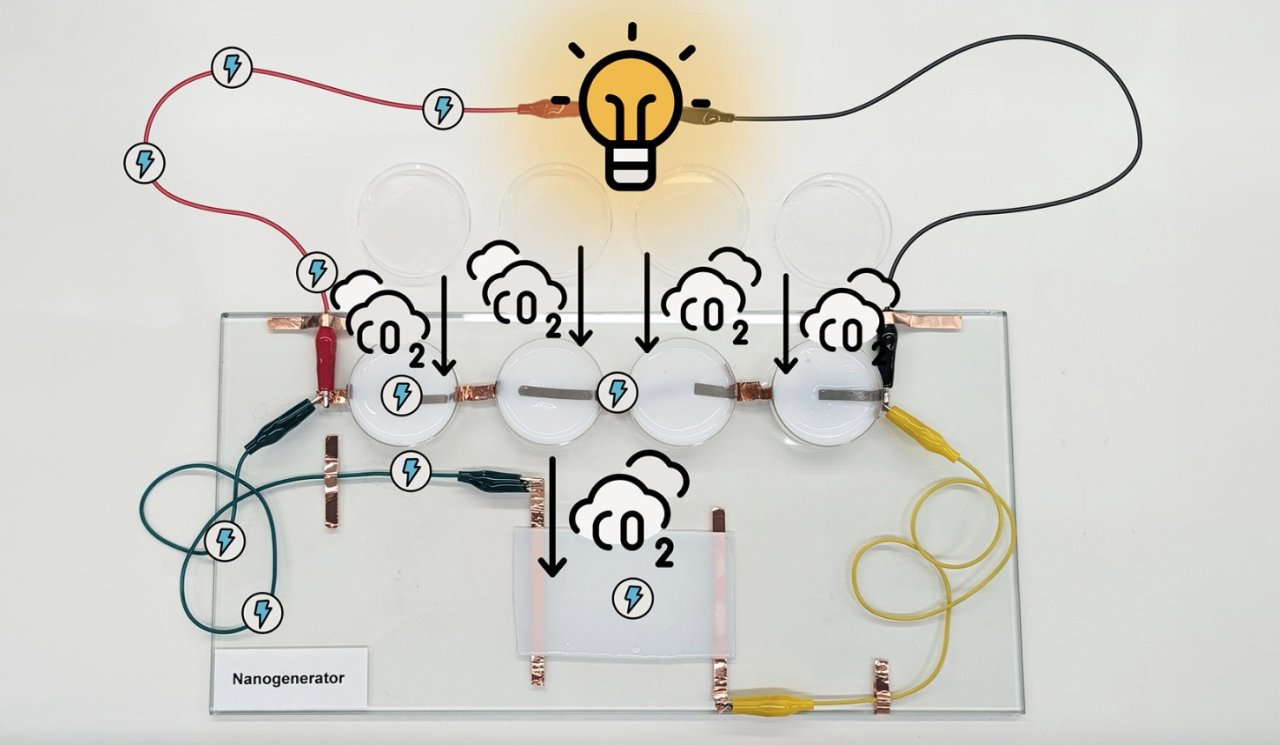
Researchers at the University of Queensland, Australia, have built a nanogenerator capable of producing electricity from CO2. Or more precisely from the chemical adsorption of this molecule. The result achieved promises several applications in the real world. As Professor Xiwang Zhang, co-author of the study published in Nature Communication, explains, a slightly larger and portable device could be built to generate energy from atmospheric CO2 and power a mobile phone or laptop. “A second, much larger scale application would integrate this technology with an industrial carbon capture process,” Zhang points out.
The chemical adsorption of CO2
But to understand the extent of innovation, we need to take a few steps backward. Chemical adsorption is a process by which a gas, in this case, carbon dioxide, is absorbed into a compound – usually a liquid with a strong affinity for the molecule itself – by binding it chemically. In other words, this is what happens in CCS plants that use aqueous adsorbents such as alkanolamines or amines to seize carbon dioxide from industrial fumes. What perhaps not everyone knows is that it is an exothermic reaction, which is that it releases energy. How to collect it? This is where Australian research comes in. The team of scientists decided to take inspiration from the biological processes of energy conversion that depend on the transport of ions regulated in biochannels.
As a result of the absorption of CO2, the amino solutions generate positively charged amino ions and negatively charged bicarbonate ions. By successfully separating them, they can be used for electricity generation. The problem with such an approach lies in the size: amino ions and bicarbonate ions are almost similar in molecular size and well mixed, making separation difficult.
Change the size
“We figured out how to make positive ions much bigger than negative ions,” says Dr Zhuyuan Wang. “And because the different sizes move at different speeds, they generate a diffusion current that can be amplified in electricity to power bulbs or any electronic device”.
The key to this work lies in a new nanogenerator composed of a “traditional” polyamine gel (already used in CCS) and a skeleton of boron nitrate often a few atoms that generate positive and negative ions. The two components were incorporated into a 90% water hydrogel, cut into 4 cm discs and small rectangles and then tested in a sealed box filled with carbon dioxide.
“We can currently collect about 1% of the total energy transported intrinsically by gaseous CO2 but, as with other technologies, we now need to improve efficiency and reduce costs,” said Dr Wang. “So far CO2 has been seen as a problem to be solved – said Professor Zhang – but it can be a resource for the future”.