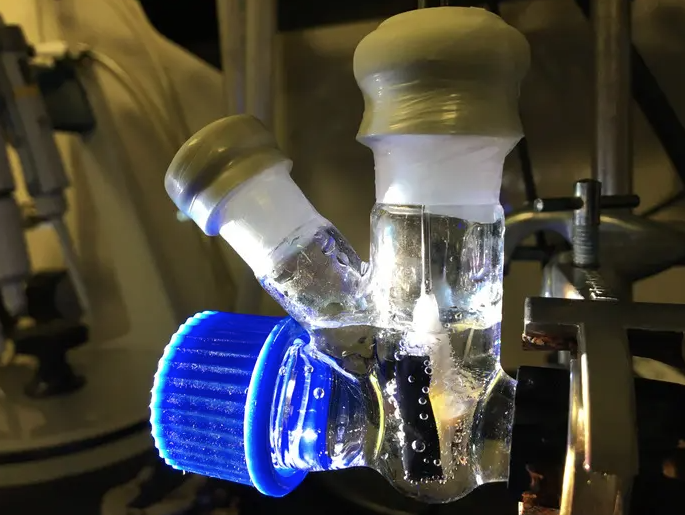
Cambridge University researchers harnessed the power of photosynthesis to convert carbon dioxide, water and sunlight into liquid multicarbon fuels in a single step
How to produce pure ethanol and propanol with artificial leaves
Sun, water, CO2. These few ingredients are enough and the artificial leaf, created by chemists of the University of Cambridge, produces pure ethanol that can be added directly to gasoline to fill up cars. Professor Erwin Reisner and his team in the generation of solar fuels achieved a world premiere.
The synthesis of liquid fuels with high energy density from carbon dioxide and water is a particularly prolific field of research. To date however, the construction of photoelectrochemical devices for the direct production of liquid fuels multi-carbonium remains an open challenge. Today, the more advanced ones – called artificial leaves – are able to produce only simple chemicals, such as syngas, a mixture of hydrogen and carbon monoxide. In other words, it takes more steps to get to the final product.
The artificial leaf of the Reisner team, on the other hand, is able to directly produce multi-carbonium liquid fuels without the need for the intermediate phase of syngas production. The secret of success is a new copper and palladium based catalyst, optimized by scientists to allow their leaf to produce more complex chemicals, in particular ethanol and n-propanol. Both alcohols are energy-dense fuels that can be easily transported and stored.
At the moment, the device constitutes only a feasibility test and shows rather contained efficiencies (~ 7.5%). But the group is working to improve the light absorbers and the catalyst in order to increase the yield. Further work will also be needed to make the device scalable so that it can produce large volumes of pure ethanol. The research was published in Nature Energy.